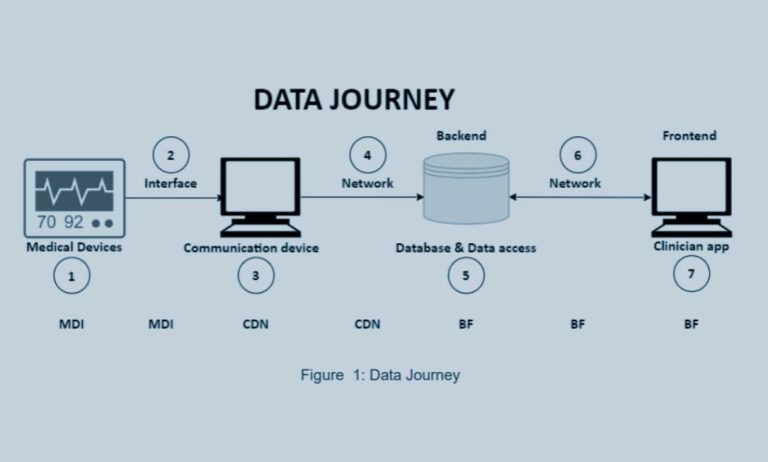
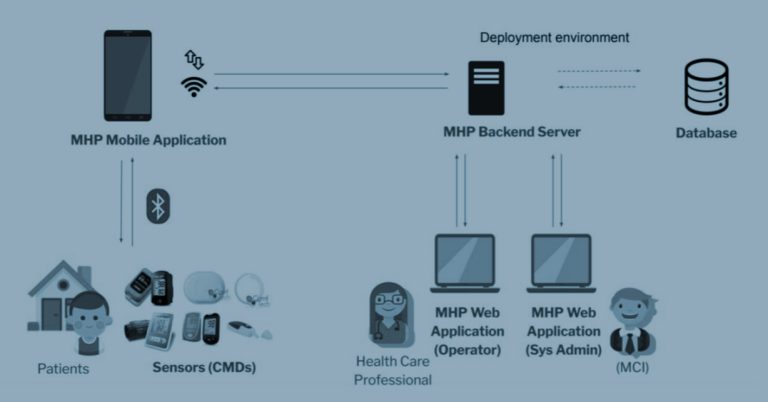
Navigating Legal and Ethical Dimensions in CYLCOMED: A Comprehensive Study
In the dynamic realm of medical technology, ensuring cybersecurity while adhering to stringent legal and ethical standards is paramount. This is one of the focuses of the CYLCOMED project, specifically targeting the cybersecurity of medical devices and the obligations of stakeholders to facilitate compliance.
Understanding the Frameworks
CYLCOMED operates at the intersection of several critical domains: privacy and data protection, medical devices, and artificial intelligence (AI). With a thorough analysis of the frameworks governing these areas, the project emphasises the adherence to GDPR standards in the project’s design and deployment. Additionally, it aims to address the legal and ethical challenges related to involving children in clinical studies, underscoring the importance of safeguarding vulnerable populations.
Key Themes and Regulatory Focus
A recent deliverable titled, “Analysis of Ethical, Legal and Data Protection Frameworks” has been produced and structured around six key themes relevant to the CYLCOMED ecosystem:
1. Privacy and Data Protection: Emphasizing GDPR compliance, this theme addresses the legal bases for processing personal and health-related data, crucial for the CYLCOMED project.
2. Regulatory Frameworks for Cybersecurity: Highlighting the complexity of cybersecurity regulations, the deliverable explores various legislative frameworks that CYLCOMED must navigate.
3. Regulatory Frameworks for Medical Devices: It discusses the specific regulations governing the safety and efficacy of medical devices, ensuring they meet the necessary standards.
4. Artificial Intelligence: Focusing on the EU’s AI Act, this section outlines the ethical principles such as human oversight, transparency, and accountability essential for developing AI-based technologies within CYLCOMED.
5. Ethics in Clinical Studies: This theme stresses the importance of upholding ethical norms, including human dignity, health protection, and research integrity, particularly in clinical trials involving children.
A Foundation for Future Guidance
The “Analysis of Ethical, Legal and Data Protection Frameworks” report is not just a standalone document but a foundational step in the ongoing legal and ethical research within CYLCOMED. It aims to provide targeted guidance to the project’s consortium partners, facilitating compliance with applicable legal and ethical principles. As the legal landscape evolves and CYLCOMED’s technical solutions develop, this deliverable will serve as a cornerstone for future studies and regulatory conclusions.
Conclusion
The comprehensive analysis presented in “Analysis of Ethical, Legal and Data Protection Frameworks” (Deliverable D2.2) is crucial for the successful implementation of the CYLCOMED project. By providing detailed insights into the legal and ethical frameworks governing medical device cybersecurity, it ensures that the consortium partners are well-equipped to navigate the complex regulatory environment. Upholding GDPR standards, addressing ethical challenges, and integrating these principles into the project’s architecture are vital steps toward achieving legal and ethical compliance in CYLCOMED’s innovative solutions.
In essence, CYLCOMED is laying the groundwork for a legally and ethically sound approach to developing and deploying CYLCOMED technologies, ensuring that the project not only meets regulatory requirements but also upholds the highest standards of ethical integrity.